Try our new line of particulate testers. Clean in Design. Complex in Theory.
Dynatek Labs is a world leader in medical device testing, equipment, services and consulting with over 35 years of experience. Our core products and services are centered around acute particulate testing; chronic particulate testing; particulates shed from catheters, balloons, guide wires, and other implantable device delivery systems; stent testing; heart valve testing; fatigue to fracture stent testing; coated device durability testing; and silicone mock vessels.
Compliant Silicone Vessels
Certified for medical device testing, optically clear, customized compliances, and customized shapes.
Mock vessels based upon your specifications:
• Shape
• Compliance
• Length
• Wall thickness
• Internal or external diameter
Dynatek Labs News
Get the latest news from Dynatek Labs and our innovations as we move forward in the medical device industry.
Dynatek Labs, specializing in the development and advancement of the medical device testing industry, offers:
Laboratory Testing
- Heart valve testing
- Stent testing
- Drug coated stent testing
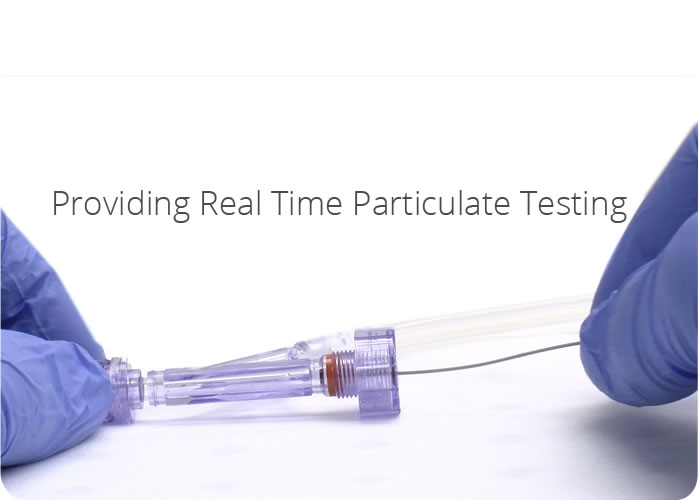
- Particle testing
- Particle capture
- Particle analysis
- Acute Particulate testing
- Chronic particulate testing
- Fatigue testing
- Protocol development
- SEM
- Microscopy
- Photo verification
- FDA standards
- ISO standards
- ASTM standards
- ANSI standards
Medical Device Testers
- Heart valve durability
- Acute particulate
- Chronic particulate
- Stent durability
- Drug eluting stent
- Particulate matter
- Fatigue to Fracture
- Catheter
- Guidewire
- Balloons
- Occluders
- VAD
- Compliance
Silicone Mock Vessels
- Straight
- Curved
- Bifurcated
- Anatomically correct
- Compliant
- Optically clear
- Custom