
Dynatek’s patented1 Acute Particulate Tester
International standards require the acute phase testing of stents, balloons, occluders, and delivery systems for particulate shedding. Dynatek has leveraged its world-class particulate analysis technology for assessing the particle-shedding behavior of these vascular devices.
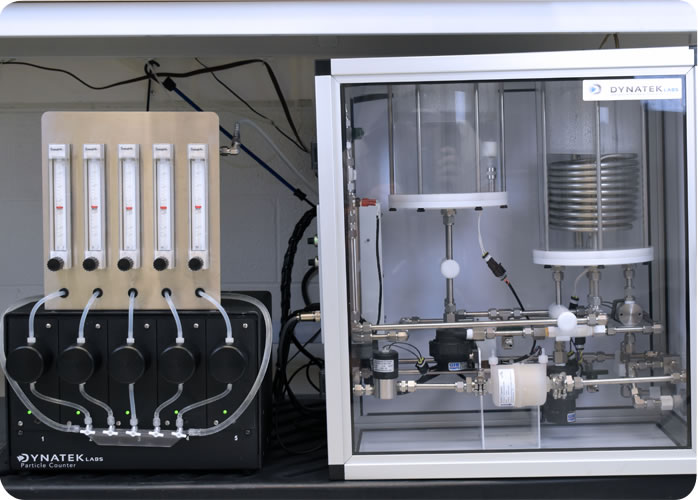
Dynatek’s innovative Acute Particulate Tester includes a temperature-controlled, closed loop into which the test sample is deployed.
The vascular device is moved down this loop until it reaches the tortuous path, a section of the test tracking fixture that is described in ASTM F2394-07, or alternatively, a tortuous path furnished by the client.
1 US Patent # 9,453,788 B2
After navigating the tortuous path, the device is advanced into a mock vessel for deployment. Particles shed by the device during tracking and deployment flow directly into a debubbler and then through custom laser-based particle counters that both count and size particles into user-defined virtual bins.
Downstream of the particle counters, the particles are captured by optional particle capture filters, for SEM/EDX/FTIR analysis, giving you critical information about the composition of the shed particles.
After particulates have been captured for further analysis, the testing fluid flows into a capacitance tank to maintain temperature, and lastly through a 0.2 micron scrubbing filter. From the scrubbing filter, fluid re-enters the flow loop, providing a continual supply of particle-free water, while minimizing waste.
The real-time particle counting advantage
Particle-shedding activity is continuously monitored throughout the duration of a sample run, and counts may be subdivided into multiple data packets based upon client preferences. This allows the user to know discrete particle counts for each phase of device advancement into the system, tracking through a tortuous path, sample deployment, and device withdrawal.
Particle data is divided into virtual bins ranging from 5-100 micron. The user may choose to up to six virtual bins with a resolution of +/-1 micron up to 50 microns, and +/- 5 micron from 50-100 microns. Particles ranging from 100-1000 micron are counted, but not sized.
Testing fluid is passed through each of the five particle counters at a rate of 100 mL/min, for a total of 500 mL/min, providing minimal down time between sample runs.

- Testing Standards relevant to the APT
- ASTM F2394-07 (2017)
- ISO 25539-2:2012
- ASTM F2743-11
- AAMI TIR42:2010
- ASTM WK46148
- ISO 12417-1:2015
- ISO/TS 17137:2014
- ASTM F3036-13
- ASTM WK60510
- ASTM WK60511
Standard Guide for Measuring Securement of Balloon Expandable Vascular Stent Mounted on Delivery System
ASTM Link
Cardiovascular Implants – Endovascular Devices – Part 2: Vascular stents
ISO Link
Standard Guide for Coating Inspection and Acute Particulate Characterization of Coated Drug-Eluting Vascular Stent Systems
ASTM Link
Evaluation of Particulates Associated with Vascular Medical Devices
AAMI Link
Standard Guide for Acute InVitro Evaluation of Drug Coated Balloons
ASTM Link
Cardiovascular implants and extracorporeal systems — Vascular device-drug combination products — Part 1: General requirements
ISO Link
Cardiovascular implants and extracorporeal systems — Cardiovascular absorbable implants
ISO Link
Standard Guide for Testing Absorbable Stents
ASTM Link
New Test Method for Simulated use testing of neurointerventional device in tortuous vasculature
ASTM Link
New Test Method for Particle evaluation and characterization for neurointerventional devices
ASTM Link
Particulate Matter Evaluation during Deployment and Withdrawal
Particulate Matter Evaluation during Deployment and Withdrawal at Dynatek Labs references the following standards and regulations:
i. ASTM F2394-07 (2017) – Standard Guide for Measuring Securement of Balloon Expandable Vascular Stent Mounted on Delivery System
ii. ISO 25539-2:2012 – Cardiovascular Implants – Endovascular Devices – Part 2: Vascular stents
iii. FDA Guidance for Industry and FDA Staff – Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems, April 18, 2010
iv. FDA Guidance – Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Angioplasty (PTCA) Catheters
v. ASTM F2743-11 – Coating Inspection and Acute Particulate Characterization of Coated Drug-Eluting Vascular Stent Systems
vi. AAMI TIR42:2010 – Evaluation of Particulates Associated with Vascular Medical Devices
vii. ASTM WK46148 – Standard Guide for Acute InVitro Evaluation of Drug Coated Balloons
viii. ISO 12417-1:2015 – Cardiovascular Implants and Extracorporeal Systems — Vascular device-drug combination products
ix. ISO/TS 17137:2014 – Cardiovascular implants and extracorporeal systems — Cardiovascular absorbable implants
x. ASTM F3036-13 – Standard Guide for Testing Absorbable Stents
xi. ASTM WK60510 – New Test Method for Simulated use testing of neurointerventional device in tortuous vasculature
xii. ASTM WK60511 – New Test Method for Particle evaluation and characterization for neurointerventional devices
Available Add-ons
• Eight-foot positive pressure flow hood to maximize working platform.
• Customized flow lines to discretely evaluate guide system particle contribution.
• Capture filter tower mounted within hood to facilitate easy capture filter replacement
• Alternative capacitance tanks and fluid heaters for elevated test temperatures.
• UPS (Uninterruptable Power Supply) system.
• Custom particle counter calibration for atypical particle size ranges.
• Capture filters to aid in chemical analysis of particles.
• Tortuous path to customer specifications; silicone or glass.
• Other custom modifications at customer request.

The flow path can be adjusted to accommodate different design purposes.
Dynatek Labs Acute Phase Particulate Tester Specification
|
Description
|
Specifications
|
Additional Information
|
|---|---|---|
|
Deployment site configuration
|
Mock vessel or custom deployment model |
|
|
Mock vessel configuration
|
Straight, curved, or bifurcated
|
Additional special configurations upon request
|
|
Typical number of flow paths
|
Up to 5
|
Customizable to accommodate various delivery accessories |
|
Testing fluid
|
PBS or distilled water
|
Addition of glycerin possible
|
|
Fluid temperature
|
Ambient to ≤ 45 ⁰C
|
Temperature control accurate to +/- 1 °C of set temperature
|
|
Flow rate (at sensors)
|
500 mL/min standard
|
100 mL/min per sensor |
|
Test monitoring
|
Pressure, temperature, flow rate, particle count and size
|
Dynatek Labs Particle Counter Product Specification
|
Description
|
Specifications
|
Additional Information
|
|---|---|---|
|
Number of particle counters
|
5 | The use of 5 particle counters reduces cleaning time of each sample or experiment to 20% of the time needed with one counter. |
|
Particle size range
|
5-900 micron | Size and count up to 100 micron Count up to 900 micron |
| Max. particle concentration |
15,000 p/mL
@ 10 microns |
Approx. 8,000 p/mL above 150 microns
|
|
Particle bin range
|
2-6 bins
|
5 bins of 5-100 micron 1 bin for 100-900 micron |
|
Particle counter calibration standard
|
USP <788>, ASTM F658 | |
| Sizing resolution | 5-50 micron: +/- 1 micron 50-100 micron: +/- 5 micron |
|
| Capture filter assembly | 47 mm diameter filter housing | Included filter allow easy swap without test interruption |
*Specifications are subject to change without notice.



