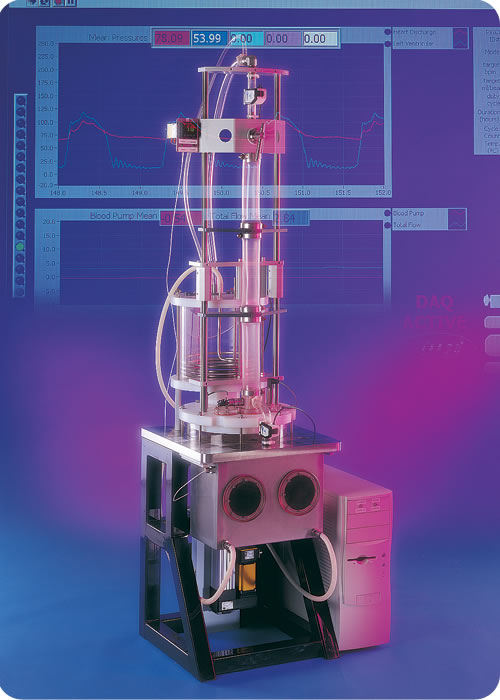
PVAD™ Pulsatile VAD Tester
The patented1 PVAD™, a pulsatile ventricular assist device tester, is a real time tester that closely simulates all the physical conditions necessary to fully test artificial hearts and assist devices.
Overview
With the use of compliant aortic sections, the fluid dynamics of this tester can emulate in vivo conditions for flow rates and pressures. The test fixture provides mechanical equivalents for the left ventricle, aortic valve, compliant aorta, atrium, and mitral valve, completing a closed cardiovascular loop. A piston attached to a linear actuator with a servomotor provides the pumping function. This tester can be adjusted to test ventricular assist devices or total artificial hearts by adjusting the volume per pulse and overall pressures and flows of the system. The sample chamber is temperature-controlled and allows the devices to be exposed to saline at body temperature for the duration of the test. The PVAD™ controlling software is written in the LabVIEW® graphical language which allows for simultaneously monitoring several conditions, managing operating parameters at programmable intervals, and collecting data from the instrumentation. Multiple testers can be linked together and operated from a central source.
Advantages of the PVAD
• Programmable for several different physiological states.
• Temperature controlled saline bath for samples.
• Can test up to three samples.
• Safety switches for temperature and pressure.
• Compliant aorta to allow for setting physiological flows.
1 US Patent # 7,018,327
PVAD Specifications
• Overall Dimensions: 18”L x 18”W x 71”H (minus control system)
• Power Requirements: 115V AC
• Maximum Fluid Displacement: 300 mL
• Number of Samples: up to 3
• Volume Capacity: 28.4 liters
• Temperature Range : ambient to 40°C
• Accuracy of Temperature: -1°C +0.3°C
• Speed: Computer controlled stroke from 1 to 120 beats per min.
• Dry Weight: 203 lbs

Implants for surgery — Active implantable medical devices — Part 5: Circulatory support devices
ISO Link



