With 31 years of experience in heart valve testing, Dynatek Labs leads the world in heart valve testing that meets ISO 5840 requirements. In addition to this decades-long experience in heart valve prosthesis testing, Dynatek Labs and our customers benefit tremendously by Dr. Jim Conti’s participation in ISO/AAMI/ASTM standards committees. Dr. Conti’s participation in the activities of the standards committees gives Dynatek Labs significant insight and understanding of the regulatory framework as it impacts heart valve prosthesis testing. Customers who have their heart valve devices tested in Dynatek’s contract testing lab benefit from understanding exactly what tests are required by regulatory bodies, and how to accomplish the test objectives in the most cost-effective manner.
The contract testing lab at Dynatek can test all heart valve designs including percutaneously-delivered valves. We can also test septal defect closure devices, artificial heart bladders, membranes/sheets, sutures/clips and intra-aortic balloons on the M6 Heart Valve Tester.
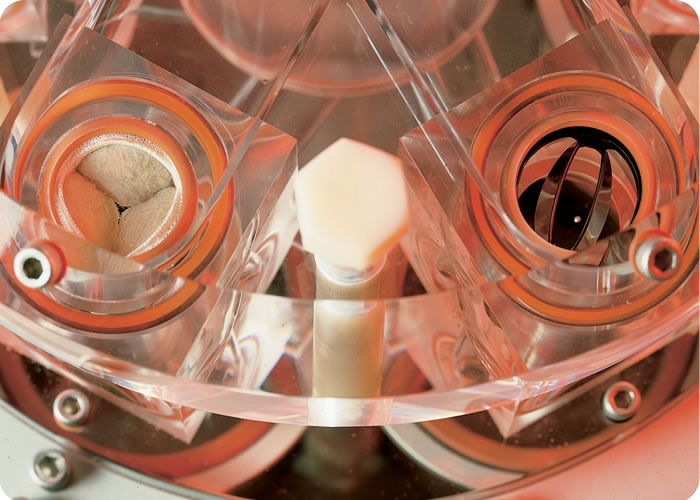
The M6 has been the gold standard in heart valve durability testing for nearly 30 years and can test from two to six heart valves or cardiac prostheses at a time. All four cardiac valves, the tricuspid, pulmonary, mitral and aortic valve can be tested on the M6 Heart Valve Tester. Dynatek Labs can test valves made of virtually any material on the M6 Heart Valve Tester.
The following tests conforming to ISO 5840 series- Cardiovascular Implants –Cardiac Valve Prostheses, and FDA Draft Guidance for Industry and FDA Staff: Heart Valves – Investigational Device Exemption (IDE) and Premarket Approval (PMA) Applications 2010 are performed at Dynatek’s contract testing lab:
Accelerated Wear Testing (AWT) or Valve Durability Testing:
AWT or valve durability testing is also referred to as valve fatigue testing and is performed on Dynatek’s M6 Heart Valve Tester. The M6 can test from two to six valve prostheses at a time. Our contract testing lab is equipped with M6 Heart Valve Testers, stroboscopes, high-performance pressure transducers, still and video cameras, and high speed cameras to carry out heart valve substitute testing under all conditions described in the standards.
For rigid or mechanical valves, valve prostheses are tested on the M6 for 400-600 million cycles, and flexible leaflet prostheses are tested for 200 million cycles with closing pressures between 125 and 150 mm Hg, according to FDA recommendations. Dynatek can work with your protocol or assist you in developing a test protocol for accelerated wear testing of your heart valve substitute.





