Scope of Services
The stent will be examined under the metallurgical microscope, and high-resolution still images will be captured by the camera and stored on the computer for further analysis.
A. Baseline Imaging of Coating Integrity
Appropriate surfaces of the stent will be examined under the microscope before it is deployed in a test platform, to establish a baseline for comparison to the stent’s coating characteristics after testing has been completed. A visual assessment of a representative stent will be performed after the stent is expanded in air (not in a mock vessel) to its maximum labeled diameter.
B. Simulated Use Imaging of Coating Integrity
Appropriate surfaces of the stent will be examined under the microscope after testing is completed. A visual assessment will be performed after the stent is removed from the mock vessel, allowed to expand in air to its maximum diameter, rinsed and dried. The location of any defects or anomalies found after the stent has undergone testing will be recorded visually and recorded. Major design features of the stent such as curves, loops, etc., will be examined for the presence of any defects or anomalies.
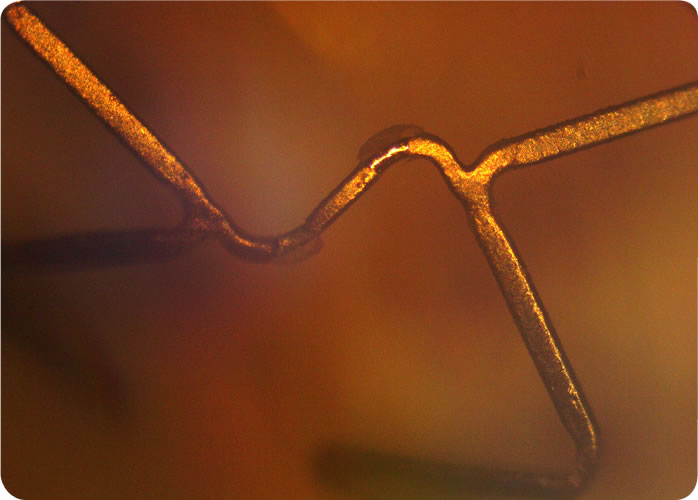
Image Analysis
Microscopic analysis of the stent will include Panoramic and Detailed Assessments. Both of these assessments will be conducted as part of the Baseline Imaging and Simulated Use Imaging of Coating Integrity:
1. Panoramic Assessment:
Ten images of the abluminal surface of the stent will be taken to assess the overall condition of the stent. The ten images will be taken from across the entire length of the stent, capturing every major design feature of the stent, such as struts, curves, loops, bends, etc., by rotating the stent incrementally. Panoramic imaging will be used to detect and locate suspected irregularities and will be taken at low magnification. During the panoramic assessment, representative visible surfaces will be inspected and the location and characteristics of coating anomalies, defects or artifacts will be noted and documented for further analysis under Detailed Assessment. Panoramic Assessment will be conducted before and after the stent is tested.
2. Detailed Assessment:
Any defects or other surface anomalies on the abluminal surface observed during the panoramic assessment will be inspected under an appropriate higher magnification (~200X, per ASTM F2743-11). The overall condition of the coating will be documented by a sufficient number of representative images so that an assessment of coating consistency can be made of the stent. A minimum of ten detailed images will be made of the stent, and more will be made to document coating anomalies and defects as necessary. Detailed Assessment will be conducted before and after the stent is tested.
3. Coating Anomalies will be Classified under Four Categories:
Category A: Irregularities involving increased or decreased thickness of coating (e.g., cracks, raised spots and depressions in the coating)
Category B: Anomalies involving nonhomogenous coating (e.g., craters in the coating, wrinkles, blisters)
Category C: Anomalies involving coating displacement (e.g., fragments breaking off, webbing, coating ‘bridges’ between stent elements)
Category D: Anomalies involving the structure of the stent and its components (e.g., broken struts)
4. Analysis Elements:
1. Baseline Imaging before testing: 10 images in Panoramic Assessment and 10 in Detailed Assessment.
2. Post test coating Integrity imaging: 10 images in Panoramic Assessment and 10 in Detailed Assessment.
3. Report





