First to develop fatigue to fracture testing of stents.
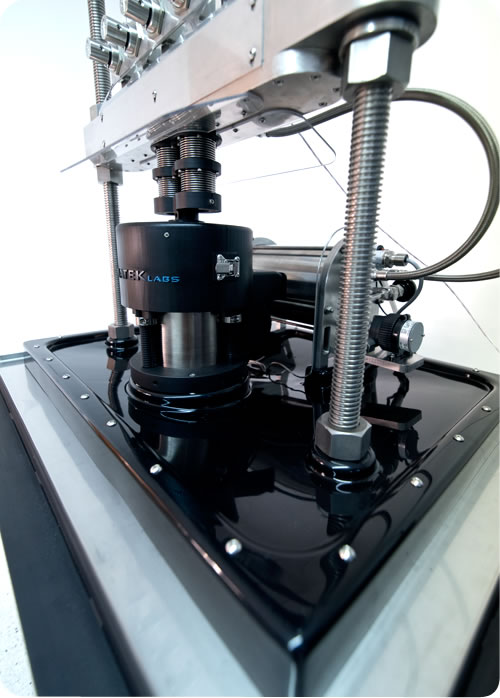
Fatigue to Fracture has been a topic of regulatory and International Standards committees for 15 years. Dynatek now has its FtF instrument (pat. pend.) that uses custom mock arteries and a robust radial durability tester to quickly and easily address the generation of data to predict endurance limits and FEA verification. The FtF allows an engineer to design a sequential stress versus failure diagram, such as a Cooper diagram, to gain insight into the long term reliability of a device. As an example, a stent design that has passed 400 million cycles of stress can be tested to failure in as little as 20 minutes at 50 Hz. This would represent the starting point of an endurance evaluation.
Learn more about the Fatigue to Fracture
First to develop technology for integrated, real-time acute particulate testing.
After 8 years of evaluating particulates shed from medical devices undergoing accelerated durability testing, it became clear to the regulatory agencies and standards committees that the introduction of particulates into the vasculature during the use of catheters, guidewires or devices was a concern. Dynatek has the first and only commercially available Acute Particulate Tester (US Patent # 9,453,788 B2). This equipment includes a fluid management system, 5 channel laser obscuration particle analyser, 4 foot or 8 foot clean hood, adjustable flow pathway, debubbler and a choice of torturous pathways. All equipment destined for the human vasculature can be evaluated.
Learn more about the Acute Particulate Tester
First commercially available coating durability tester with in-line particulate analysis.
While attending ISO meetings, it became apparent that the analysis of particulates shed from implanted medical products would soon become vital. Dynatek personnel were tasked to evaluate the best experimental protocols and equipment to fulfill this need. After training and experimentation, Dynatek chose “in line” laser obscuration particle analysis that eliminated the serious problems associated with a “bucket” method. To this day the in line technique is still state of the art.
Learn more about the Coating Durability Testers
First to develop precise high-speed photographic verification protocols and procedures.
High speed photographic verification was first commercially used by Dynatek to fulfill customer needs to monitor the motion of implantable products such as stents during high speed testing. Research published1 by Dynatek demonstrated that the external cyclic distension of a mock artery containing a stent is substantially different than the cyclic distension of the stent. The application of these results is the recently published set of protocols that Dynatek generated to utilize this technology. Most current International Standards now require that high speed durability testing of implantable devices show strains equivalent to those during physiologically relevant speeds.
1Mock Artery Distension: Comparison of Optical, Mechanical and Theoretical Results
Biomedical Science Instrumentation, 43, pp. 46-53, (2007)
Learn more about the High Speed Photographic Verification of Stent Deflection
First to offer a commercially available pulsatile artificial heart tester.
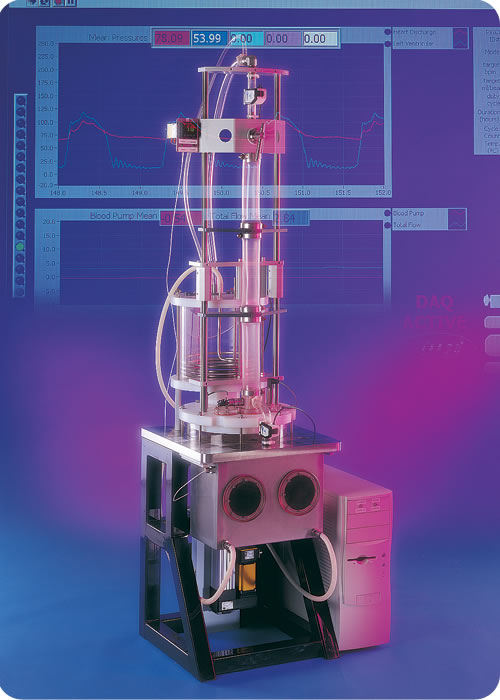
A commercially available pulsatile ventricular assist device (LVAD) durability and performance tester was first introduced by Dynatek. To our knowledge, this is still the only commercially available pulsatile circulatory support device tester in the world. Outfitted with silicone arteries and peripheral vessels as well as a computer controlled ventricular actuator, the PVAD line of tunable testers already has over 175,000 hours worth of testing to prove its reliability. With modular vascular resistance and compliance elements along with the tunable ventricle, the PVAD can attain virtually any pulsatile cardiovascular condition that might be needed to evaluate a circulatory assist device, whether it be for bridge to transplant or destination therapy.
Learn more about the PVAD Pulsatile VAD Tester
First to use 5% to 7% compliance for mock vessels.
5% to 7% dynamic internal compliance is a number that is widely used internationally to produce vascular replicates into which implantable cardiovascular products can be deployed for testing. Fifteen years ago, Dynatek presented research to the NHLBI and undertook a major review of all found publications involved with the compliance of natural vessels either in vivo or in vitro. Dynatek suggested an average number of 5% to 7% change in diameter per 100 mm Hg internal pressure at 72 BPM to reasonably represent those conditions found in vivo . Although somewhat still controversial, most laboratories internationally now use this target. Data generated in laboratories all over the world can now be compared to each other because of the utilization of this common compliance for the testing.
Learn more about the Compliant Silicone Vessels
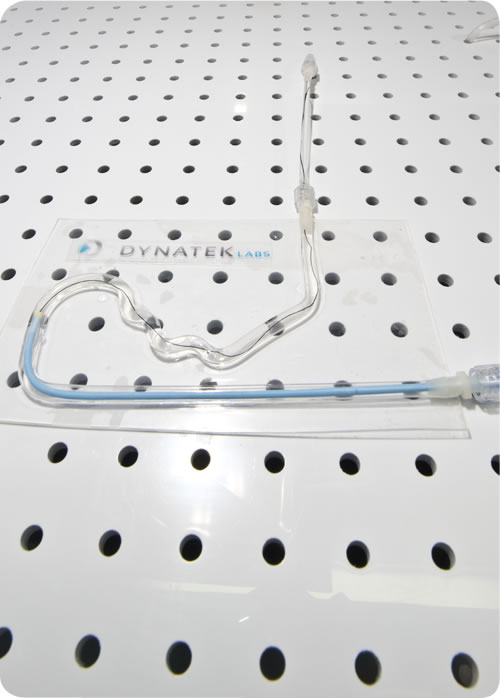
First in the use of precise, optically clear mock vessels.
Dynatek was responsible for the first introduction of vascular mechanical models to test the performance and durability of products and devices that would be implanted into or used within the human vasculature. Dynatek now supplies models in clear silicone. The mechanical properties of the starting materials and the resultant mock arteries or models can be tuned along with their dimensions. Dynatek uses a variety of technologies including CT scans, MRIs, and SolidWorks stl files to generate dipping mandrels that are produced using a patented process that yields enormous versatility in producing silicone models.
Learn more about the Compliant Silicone Vessels
First to use tuned fluid oscillation in heart valve testing.
After more than 30 years, the M6 is still considered the international gold standard in heart valve testers. The M6 has, at one time or other, been used to test nearly every heart valve available for implant today. Pioneer Professor Milton Swanson (our founder), considered by many to be the world’s first true cardiovascular fluid dynamicist, introduced this instrument using a combination of a bellows volume drive with tuned fluid oscillation. The heart valves are opened and closed fully while attaining targeted closing pressures without the need to completely stop a column of liquid. The systemic pressure of the M6 can be raised to reduce the occurrence of cavitation during high speed testing. The M6 can be used to evaluate a variety of films, candidate leaflet materials, septal repair products and LVAD bladders and all heart valve designs.
Learn more about the M6 Heart Valve Tester
First to create velocity flow standard.
Dynatek introduced the first and still the only portable pulsatile velocity flow standard in the world. Most other devices can only produce an accurate flow rate, or volume per minute. The EchoCal produces a highly reproducible pulsatile sine wave accurate to within 2 1/2% of the set velocities. Far more important than simple flow studies, velocity is a number that determines such characteristics as cavitation, Doppler measurements and velocity versus time characteristics of pumping systems. This instrument can be used to calibrate or check the accuracy of any flow measuring systems that utilize velocity as their fundamental measurement, such as laser Doppler anemometers, medical ultrasound machines or any other velocity measuring device.
Learn more about the Benefits of Accurate Doppler Ultrasound
First to introduce Dynamic Internal Compliance of mock vessels.
In the evaluation of vascular grafts and/or products that go into the vasculature, Dynatek was the first to realize that one of the most important mechanical characteristics is the dynamic internal compliance. Previously, only external measurements were taken and often were acquired in a static mode. After a thorough literature search and years of research, Dynatek not only demonstrated the errors inherent in external measurements, particularly of a static nature, but designed instruments and protocols to evaluate this critically important characteristic.
Learn more about the DCT Compliance Tester
First commercially available stent/graft durability tester with single ended pressurization.
Thirty years ago Dynatek introduced the SVP (Small Vascular Prosthesis Tester), which quickly became the instrument of choice in most vascular graft development laboratories. The key to the SVPs success was the adoption of fundamental fluid dynamic principles that allowed ultra fast testing without turbulence. In addition, by combining electroplated bellows and robust mechanical engineering designs, the SVP paved the way for vascular device durability testing.
Learn more about the SVP Stent Tester