Current Trends in the Durability Testing of Medical Products
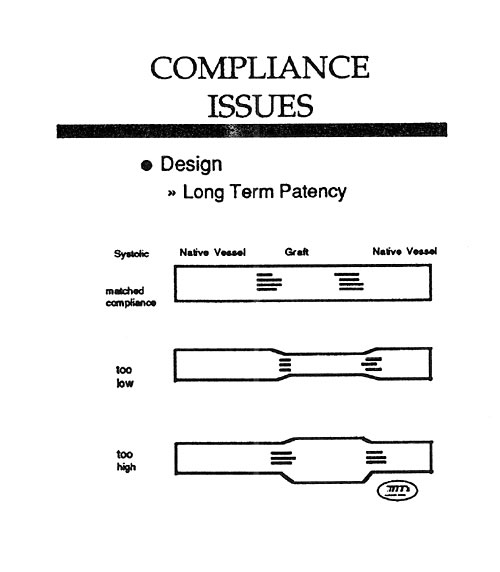
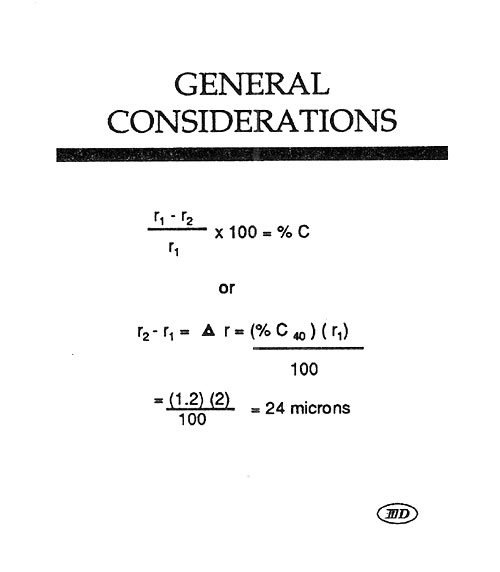
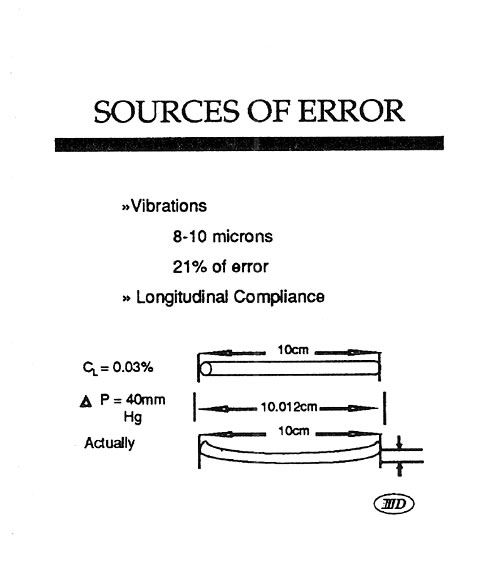
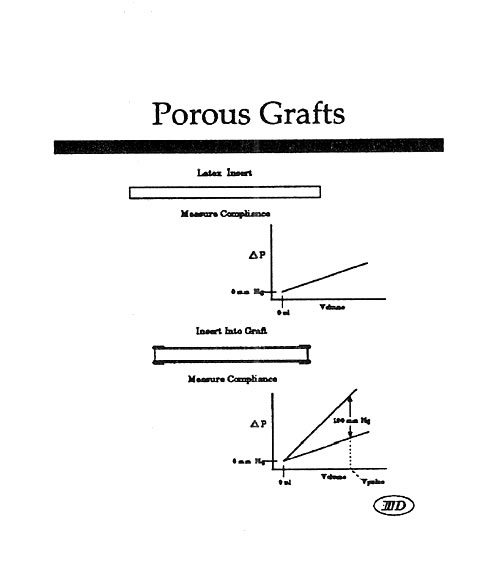
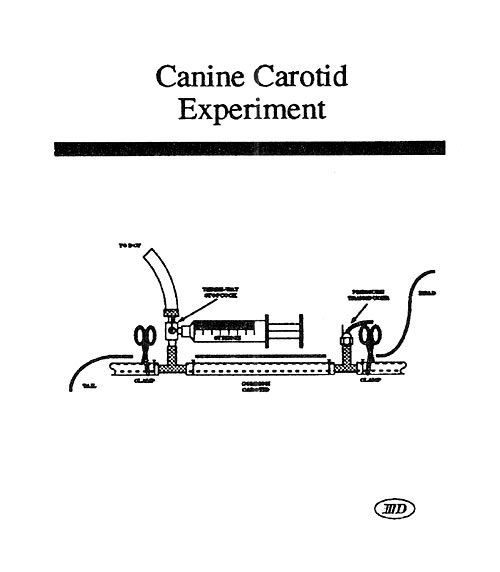
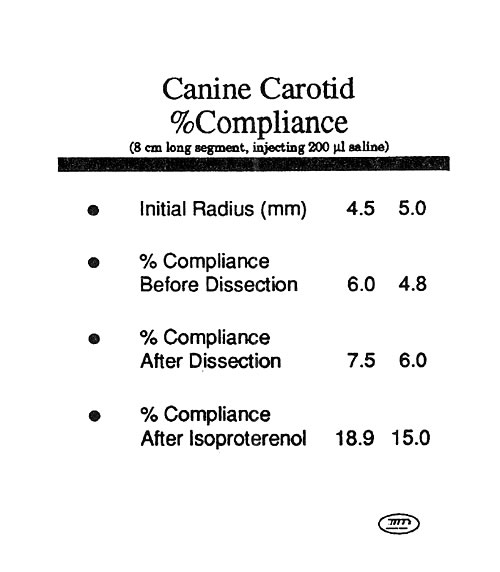
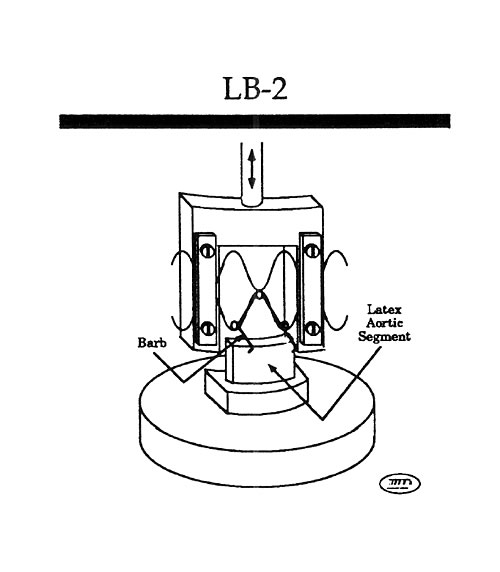
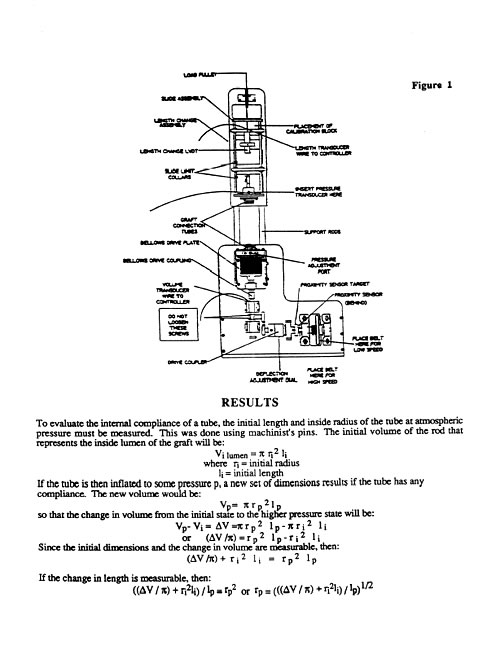
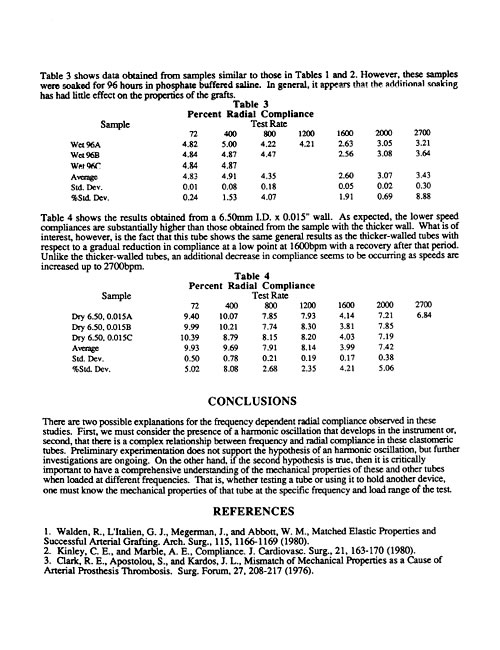
Mock Artery Compliance
by | SFB Workshop 1998 | Publications, Compliance
Current Trends in the Durability Testing of Medical Products
WORKSHOP
J.C. Conti1, E.R. Strope
Dynatek Dalta Scientific Instruments, P.O. Box 245, Galena, MO 65656
1 Southwest Missouri State University, Dept. of Physics and Astronomy, 901 S. National Ave., Springfield, MO 65804-0094
SFB Workshop 1998
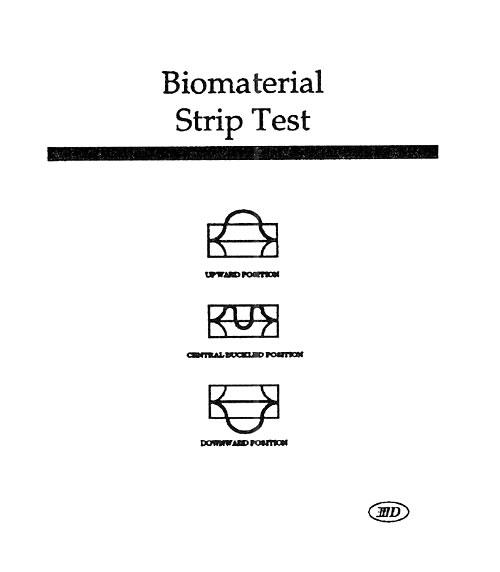
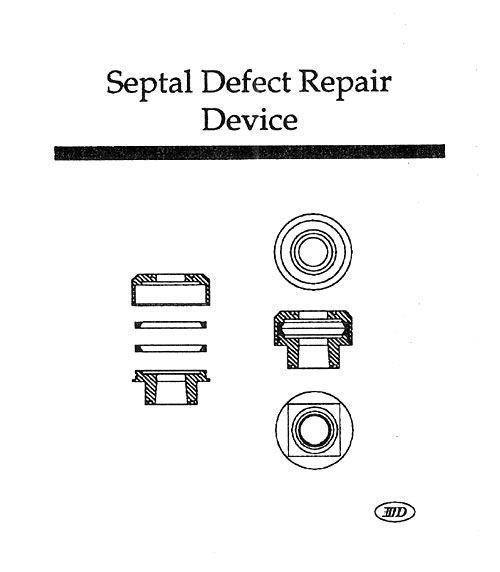
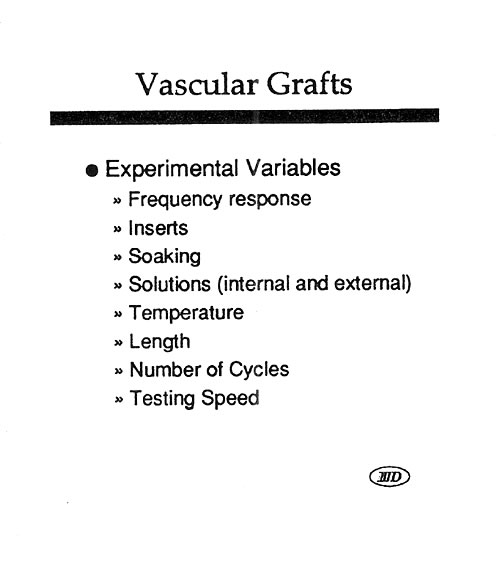
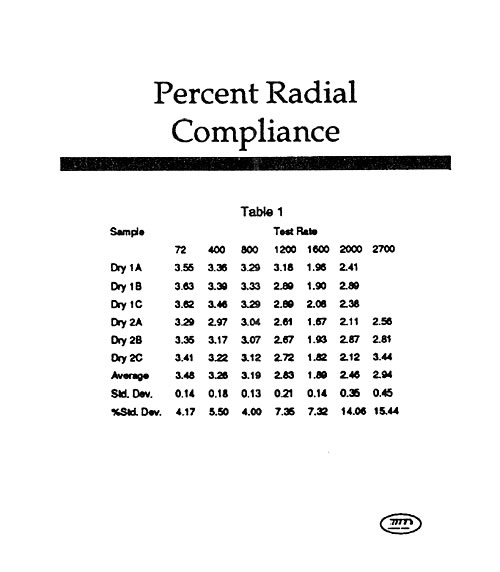
Last year’s workshop covered the history and more recent approaches used to evaluate the durability of a variety of medical products in vitro. The general theory and approach to high speed testing was reviewed. This year’s workshop will focus primarily on those testing techniques that have been developed or upgraded during the last twelve months. In particular, the latest approaches to the testing of stents, stent/grafts, septal defect closure devices, bifurcated grafts, left ventricular assist devices, adhesive bonds, and packaging will be reviewed. This workshop will give attendees a broad understanding of the issues that must be considered when designing and carrying out high speed dynamic testing, as well as very recent information uncovered during the last year with regard to individual product types. The Dynatek Biomaterials Society Workshop has a reputation for minimal commercialization and maximal technical training.